I have an idea for a new project
>So it would be a ten gallon fish tank planted heavily with peace lilies and D. maculata
>the substrate would just be peat moss (no cap)
>there would be two bunocephalus cats
>no filtration just an air stone
my question is how feasible would this be? It would be full of plants in conjunction with the bacs living in the moss and in their roots would take care of the bioload. also I feel like the cats would love the peat for digging and hiding.
Only issue I have is pH, peat has low TDS so it should be fine in that respect.
how much would using peat as substrate screw with my pH I know the tank would have a very black water.
any input would be appreciated
-regards,
Nico
Peat moss Walstad method
- pleco_breeder
- Posts: 892
- Joined: 09 Dec 2003, 16:51
- My articles: 2
- My cats species list: 17 (i:0, k:0)
- Location 1: Arizona
- Interests: breeding plecos and corys
- Contact:
Re: Peat moss Walstad method
I've done similar in the past with used/depleted peat from my blackwater treatment barrel. If using fresh peat, the pH swings would likely be too much for most fish to handle with even a small water change. Likewise, the bacteria will be greatly reduced due to the lower pH in the tank and you won't have much of a bacterial balance at all.
Larry
Larry
Impossible only means that somebody hasn't done it correctly yet.
-
Mike_Noren
- Posts: 1395
- Joined: 25 Jul 2003, 21:40
- I've donated: $30.00!
- My articles: 1
- My images: 37
- My cats species list: 5 (i:0, k:0)
- Spotted: 9
- Location 1: Sweden
- Location 2: Sweden
Re: Peat moss Walstad method
This might interest you: http://gabhar.wordpress.com/2010/12/29/ ... ater-tank/
-- Disclaimer: All I write is strictly my personal and frequently uninformed opinion, I do not speak for the Swedish Museum of Natural History or FishBase! --
Re: Peat moss Walstad method
Thank you for the link
I think I am going to give this is a try. I will probably put a HOB on it and plant it with emmersed and immersed plants. The only issue at this point would be the pH maybe I can boil my substrate....
I think I am going to give this is a try. I will probably put a HOB on it and plant it with emmersed and immersed plants. The only issue at this point would be the pH maybe I can boil my substrate....
-
dw1305
- Posts: 1079
- Joined: 22 Oct 2009, 11:57
- Location 1: Corsham, UK
- Location 2: Bath, UK
- Interests: Natural History, Ecology, Plants, Biotopes, Taxonomy, Nitrification, Cricket & Northern Soul
Re: Peat moss Walstad method
Hi all,
I've done this, it works quite well with "white peat", and less well as the peat becomes darker and more decomposed and the fragments smaller. If you can obtain it you need something like "Tref Baltic Peat" for the substrate.
Because sphagnum moss peat is harvested from rain-fed peat bogs ("ombrotrophic mires") all the cation exchange sites are filled with protons (H+ ions), which are exchanged for other cations according to the lyotropic series. Sphagna are very efficient at extracting other cations, and can produce a Sphagna mono-cultures where no other species can survive because of the non-availability of other cations. The figure for intact peat bogs in the UK is about 2% of the exchange sites have monovalent cations (other than H+) and a slightly higher percentage have multivalent cations retained, but you are still talking about over 90% of the exchange sites having a H+ ion present.
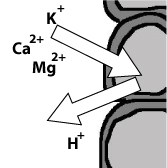
You get exactly the opposite effect in hard alkaline buffered water (like Lake Tanganyika), where the pH is extremely stable and you need a huge change in water chemistry to alter the pH at all.
cheers Darrel
I've done this, it works quite well with "white peat", and less well as the peat becomes darker and more decomposed and the fragments smaller. If you can obtain it you need something like "Tref Baltic Peat" for the substrate.
Don't boil it, the ability to soften water is dependent upon the intact cells in the sphagnum itself, these carry on functioning after the moss has died. The cells themselves are rich in phenolic compounds (that reduce the rate of decomposition, even in aerobic conditions), and there is a good correlation between the content of "unesterified polyuronic acid" and the cation exchange capacity.pH maybe I can boil my substrate
Because sphagnum moss peat is harvested from rain-fed peat bogs ("ombrotrophic mires") all the cation exchange sites are filled with protons (H+ ions), which are exchanged for other cations according to the lyotropic series. Sphagna are very efficient at extracting other cations, and can produce a Sphagna mono-cultures where no other species can survive because of the non-availability of other cations. The figure for intact peat bogs in the UK is about 2% of the exchange sites have monovalent cations (other than H+) and a slightly higher percentage have multivalent cations retained, but you are still talking about over 90% of the exchange sites having a H+ ion present.

This is a common misconception, and relates to the nature of the pH scale. pH is purely a ratio, and it tells us nothing about amounts. The easiest way to think about this is to think of as:If using fresh peat, the pH swings would likely be too much for most fish to handle with even a small water change.
We've all ready seen that sphagnum peat will lower pH (by donating H+ ions during cation exchange) and also conductivity (again by cation exchange, by replacing a metal ion that is an electrical conductor with an H+ ion that is an electrical insulator). As we approach pure H2O (what we normally call water isn't H2O, but a dilute solution of various salts), any small addition of acids or bases will cause huge changes in pH. This doesn't effect soft water fish, they live in an environment where the pH will fluctuate over several orders of magnitude during the day as CO2 and O2 levels change, these changes don't effect the alkalinity or water chemistry, purely the pH.acids as H+ ion donors and bases are H+ ion acceptors
You get exactly the opposite effect in hard alkaline buffered water (like Lake Tanganyika), where the pH is extremely stable and you need a huge change in water chemistry to alter the pH at all.
This is relevant, all biological processes are slowed, you can see this in the very slow decomposition of dead leaves etc in the tank, but if you are heavily planted with suitable plants (like in Mike's link) this shouldn't be a problem.Likewise, the bacteria will be greatly reduced due to the lower pH in the tank
cheers Darrel




