Article © Bruce Brethauer, uploaded January 01, 2002.
The Royal Farlowella is a striking fish; at first glace it is all angles, with large triangular pectoral and dorsal fins and with triangular heads coming to a point in a barely upturned rostrum ("nose"). The body tapers severely to a scant few millimeters at the caudal peduncle. The tail is crescent shaped with each point trailing a filament to perhaps three inches (7.5 cm.) in length. The markings, while rather bold, are in somber colors of browns, near blacks and olive. The markings continue into the tail filaments which bear zebra-like alternating bands of olive and a very nearly black dark brown. To me, the appearance of these fishes are reminiscent of the fossil armored "fishes" of past epochs. In addition, their curious appearance, dark coloration, and their generally inactive habit may suggest to some people that this fish may have a habit similar to the ambush predators; but contrary to their appearances, I have found these fish to be one of the most suitable fishes for the community aquarium. Except when defending its eggs from other fishes, I have never seen it become in any way aggressive with other fishes; and even when other fish in the aquarium spawned, they harmed neither the eggs nor the fry. As with many fishes from similar genera, it can be difficult to identify the exact species, as there are several species which are similar in appearance. Oftentimes, the exact identification of the species can only be determined after making close examinations of the scutes on the fishes underside, and to note the location of odontodes on the male fishes. I have provided a photograph of the underside of the male fish to illustrate the scutes (see this page) the colors in this image are unnatural, as I have modified the image in photoshop to highlight the boundaries of the scutes. The male fish produces a thick growth of odontodes along its "cheeks".
When I acquired my first two fishes of this species, I had done so with the hope of breeding them.I had already had some success in breeding Farlowella vittata (see this article), but finding and identifying female fishes of this species has proven to be fairly difficult. While one of my male fishes maintained a dense growth of odontodes along his "cheeks" continually since I had first acquired him over one year ago (see this page), the absence of odontodes will not definitively identify female fishes, as some males will periodically loose these when they are out of breeding condition. The only reliable way to properly sex these fishes is to examine the genital papilla: in female fishes, these are short conic protuberances in a noticeably recessed depression. When spawning, the papilla of female fishes develops into a short tube or ovipositor (see this page). Contrary to usual descriptions of male fishes, the genital papilla of my fish does not bulge noticeably, but is marginally depressed with only the slightest bulging about the anus (see this page). Female fishes may also have somewhat fuller bodies, but this trait can be subtle, and is not a reliable means to distinguish between the sexes. Imported fish are more often than not, male. Several possibilities may explain the preponderance of male fishes in the trade: males may tend to have longer, more developed filaments on their tails making these more desirable to collectors, and therefore more desirable to importers, there is speculation that the ratio of male fishes to female fishes is quite high in wild populations (I have heard ratios of 4 to 1 in some species, but cannot confirm this from my readings). My belief is that males which are guarding eggs are essentially "sitting ducks" making them comparatively easy to catch in the wild.
My fish are kept in a 50 gallon aquarium which is equipped with an Aqua Clear Aquatics CAP 2200 power head. The aquarium is set up as a river tank (see this link), with a fairly modest current. Unlike my Farlowella vittata, which seem to be particularly fond of areas with remarkably strong currents, my Sturisoma seem content with the weaker flow rate provided by the CAP 2200 power head (rated at about 680 gallons (2200 liters) per hour (this flow rate is significantly reduced when the mass air input hose is attached). While this flow rate may seem substantial by most accounts, it hardly compares with the flow rate of the RIO 3100 Power Head installed in my other river tank, which despite its rating of 900 GPH (3100 LPH), seems to move three times as much water as the other pump. There is no other filtration other than that provided by the foam pads on the intakes of my water circulation manifold. I make frequent water changes, replacing slightly less than half of the water with aged tap water about once per week, I also periodically remove or siphon off the uneaten portions of the kale and collard greens upon which they are fed (more on feeding later).
At the time that these fish spawned, the temperature of the water was the water was 77°Fahrenheit (25°C), and the water was tested with the following results:
Ammonia: 0
Nitrite: 0
Nitrate: 0
pH: 7.8
KH/Alk: 4
Also at the time of spawning the other inhabitants of the tank included four "Lizard Catfish" (one of the species of hillstream loaches) a single Botia striata to keep populations of a particularly prolific pond snail under control, and a single Ancistrus catfish (one of the bristlenose plecos, the exact species is anyone's guess). I had to remove this last species as he proved to be a threat to the eggs. I removed some of the other inhabitants as I was able (they are expert at dodging the net). By the time that hatching began, I had reduced the other inhabitants to two "Lizard Catfish" and the Botia striata. My original intention was to remove all other inhabitants of the tank with the possible exception of the Botia, prior to spawning and to maintain the tank as a species aquarium, but spawning occurred before I had an opportunity to remove all other inhabitants of the aquarium. I have chosen to retain the current occupants until the fry have hatched, or until the other occupants became too interested in the eggs or fry, as I thought it the lesser of two evils to maintain the existing occupants rather than to risk excessively disturbing the male with frantic attempts to catch these fish.
The aquarium "furniture" includes a few large stones (mostly quartz and granite, and a few interesting stones of limestone / dolomite or of a similar type). The substrate consists of "pea gravel" which I acquire at a local building supply company, this comprises a variety of stone types, mostly metamorphic and sedimentary (mostly flint, limestone and dolomite). I have also provided a cork wall at the back of the aquarium, and a large piece of bogwood. The aquarium is planted with a selection of Anubias species and a crested variety of Microsorium pteropus.
The Sturisoma are primarily vegetarian: In habitat these and related species are said to feed upon algae and aufwuchs (algae, planktonic and unicellular animals which live upon the algae and substrate), I suspect that various flatworms, crustaceans and small insect larvae may also comprise a small portion of the diet of this species in the wild (I don't know if these are also considered a part of aufwuchs). I maintain my fish on a diet of collard greens and lacinato kale (also known as "alligator kale") (see this page). These are both open headed varieties of cabbage which are particularly high in their vitamin and mineral content. A local breeder of reptiles had given me this important tip: the prevailing "wisdom" of feeding lizards (or by extension, fishes) a diet of lettuce and spinach would doom the animal to a slow death by malnutrition: kale and collards in contrast, are especially high in nutrients, and make a very nearly complete diet for vegetarian species, or at least those species which feed almost exclusively on algae.
To prepare these leaves for my fishes, I would soften them by first freezing them solid in my freezer, and then plunge them in my aquarium. My feeling is that there is less loss of nutritional value in freezing the leaves than would occur in par-boiling: this treatment usually softens the plant tissues sufficiently for the adult fish to begin feeding immediately. Younger fishes with smaller, less strong jaws and teeth usually require leaves which are softer; a day of soaking following freezing usually softens the leaves sufficiently to be available to younger fishes.
I also supplement this diet with two types of sinking pellets, one balanced for the diet of vegetarian fishes, and another which contains a higher percentage of animal protein for carnivorous fishes. Both types of these prepared foods are accepted by all of the fish in this tank, although the Farlowella preferentially feed upon the collards and kale greens, and only supplement their diet with the pellets. I have never attempted to feed any of my fishes exclusively on prepared foods. On occasion, I provide the other fishes in the tank with live "California Black Worms", which appear to be a close relative to the tubifex worm. These are darker, and somewhat more robust than tubifex worms, but in all other matters appear to be much the same. Observe the same regimen of cleaning prior to feeding to fish in the aquarium, as these worms will also carry large quantities of bacteria, and may also introduce other pests into the aquarium. On rare occasions, I have observed my Sturisoma feeding on this live food, but this is quite uncommon, as a rule, my fish seem entirely content to consume the other foods provided, and seldom eat live foods, so I do not believe that providing live foods is in any way necessary to bring these fish into breeding condition. I should also mention that my fish have never devoured any of the aquarium plants: I suspect that the kale and collard greens satisfies my fishes' appetites to such a degree that the aquarium plants are regarded as starvation foods only.
Within days of observing minor indications that the female may be becoming gravid, my fishes spawned. I suspect that in the future, I will become more adept in reading the earlier signs of spawning, but not this time. Sadly, the spawning process was not observed, so I cannot provide details.
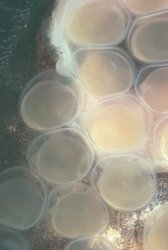
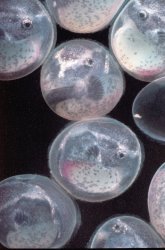
Adhesive eggs (measuring approximately 2.5 millimeters in diameter) were deposited on the glass of the aquarium about 2 1/2 inches (6-7 centimeters) from the waterline. The placement of the eggs provided an excellent vantage point to observe and photograph the daily development of the embryos. While many of my observations were made without magnification, a photographers loupe (approximate magnification of 4x) revealed a remarkable degree of detail. The eggs were deposited in a compact, roughly circular mass such that no eggs were placed on top of another. The male guarded the eggs, protecting the mass with his body, and continually fanned the eggs with rhythmic movements of his pectoral and ventral fins, (see this page) leaving them only rarely to feed. Following spawning, the female shows no interest in the eggs, and takes no additional role in their care. I did not remove the female following spawning: it appears that she presents no threat to the eggs, as she ignores these completely. The presence of the female in the aquarium does not seem to distress the male in any way. On the rare occasions when the male leaves the eggs to feed, he will feed alongside the female without any sign of conflict. I tend to take the attitude of "if it ain't broke, don't fix it" in these matters. At the first signs of difficulty, I will immediately isolate either the parents or the fry to another tank.
At hatching, the well developed dorsal, ventral and anal fins are readily apparent and a "moustache" can be observed on their mouths (see this page). The fry absorb the yolk sac within about 2 to 3 days. They become increasingly active, and are more prone to swim. At this stage, the fry initially position themselves in areas which receive fairly strong currents. This may reflect their need for highly oxygenated water, or may be a means to escape predation from other fishes which are less adapted to stronger currents. At this age, I do not detect the development of filaments on the caudal fin, or any sign of the characteristic dermal flap on the iris of their eyes, apparently this feature develops later (see this page). Within a week after hatching, the fry increasingly forage for food, and become increasingly difficult to find in the aquarium. It is important to maintain a constant supply of food at this stage, as I believe that any species of fish which is adapted to living on the bottom of swiftly moving streams generally do not store much fat in their tissues, possibly because a high percentage of fat would make these species too buoyant. Once all food in the aquarium has been consumed, fry are especially prone to starvation. It is best to provide an aquarium densely planted with a variety of established plants, I maintain a separate aquarium with a simple substratum of local gravel (flint ,dolomite etc). It is densely planted with Microsorium pteropus and various varieties of Anubias, which I attach to pebbles with rubber bands. These plants readily attach themselves to the pebbles as they grow so planting the fry aquarium is a simple matter of dropping these ferns (pebble and all) wherever they are desired. It becomes easy to plant the tank as densely or as sparsely as you may wish. Kept isolated from snails and other fish, these plants will usually support a diverse fauna and flora of their own, and may provide an important first food of aufwucks for the fry. I also try to maintain a constant supply of well softened collard and / or lacinato leaves. I provide these as soon as the fry have hatched, even though they may not consume any significant food until their yolk sacs have been completely absorbed. The fry will probably not be able to completely consume the leaf before it begins to foul the water, so keep a close eye of the kale, I usually remove any portions of the leaf which have not been consumed within 3 days. As a rule, I add small pieces of leaf every day to insure that there is a continual a supply of sufficiently softened leaves for the fry. Failure to provide a constant supply of food will quickly result in starvation.
Remarkably, the parent fishes spawned again 13 days after the first clutch of eggs had been laid, and within about 2 or 3 days of the last fish hatching from the first spawning. This clutch was located at the end of the aquarium opposite of the powerhead, approximately 6 inches (15 cm) below the waterline. This area received a fairly high degree of current from the power head, so the male typically guarded the eggs by positioning himself beside the mass rather than on top of it and spent less energy on fanning the eggs himself. The location of the mass only provided me with an oblique view of the eggs, so I have a much poorer view of their development, and made fewer photographs of this spawning. There appears to be considerably more eggs in this clutch compared to the first clutch (an estimated 100 eggs vs. about 50 in the first). It is difficult to get a good view of the egg mass, but I am guessing that the eggs were deposited in an elongated oval or triangular arrangement. In other details, this spawning was nearly identical to the first. By the time that these eggs had hatched, I had succeeded in removing all other inhabitants of the aquarium, so I have chosen to keep the fry with the parents until they have had several weeks to grow. following this, I plan to remove these fry to a separate aquarium to grow to a larger size. In several months, I hope to post an update to illustrate the growth of the fry, and to share some of my experience in raising these to maturity (it is my guess that these fry will not reach sexual maturity until this time next year).
There is further information on this species on the Cat-eLog page.
Back to Shane's World index.